Following the success of our webinar last November on Generative AI in Pharma, we organized the next one specifically for pharma regulatory affairs leaders on how to leverage Generative AI in Pharma Regulatory Affairs.

Our special guest, Scott Sawler (Former DG of Marketed Health Products Directorate, Health Canada) interacted with Gramener’s Nutan B. and Santosh Shevade on patient data privacy, GenAI, and pharma regulations.
Table of Contents
Our Webinar was Interactive!
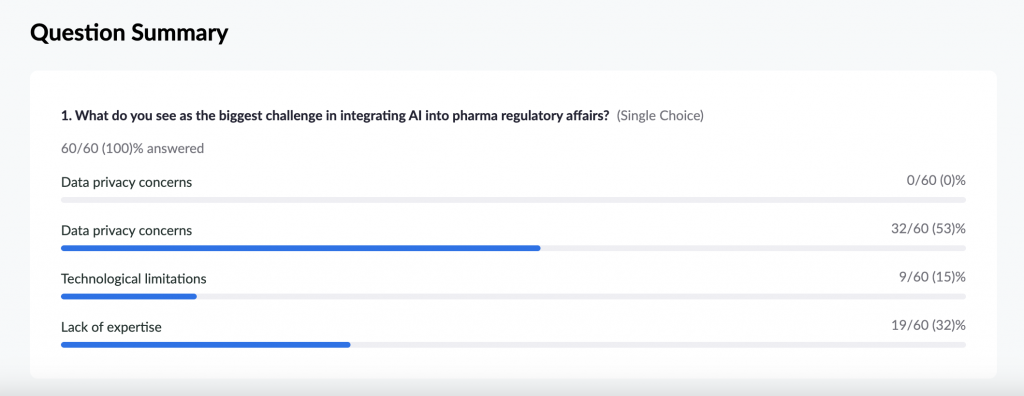
We started our webinar with interactive polls to understand the challenges of our participants. So, in the first poll, we can see that ‘data privacy concern’ is the biggest challenge for major attendees.
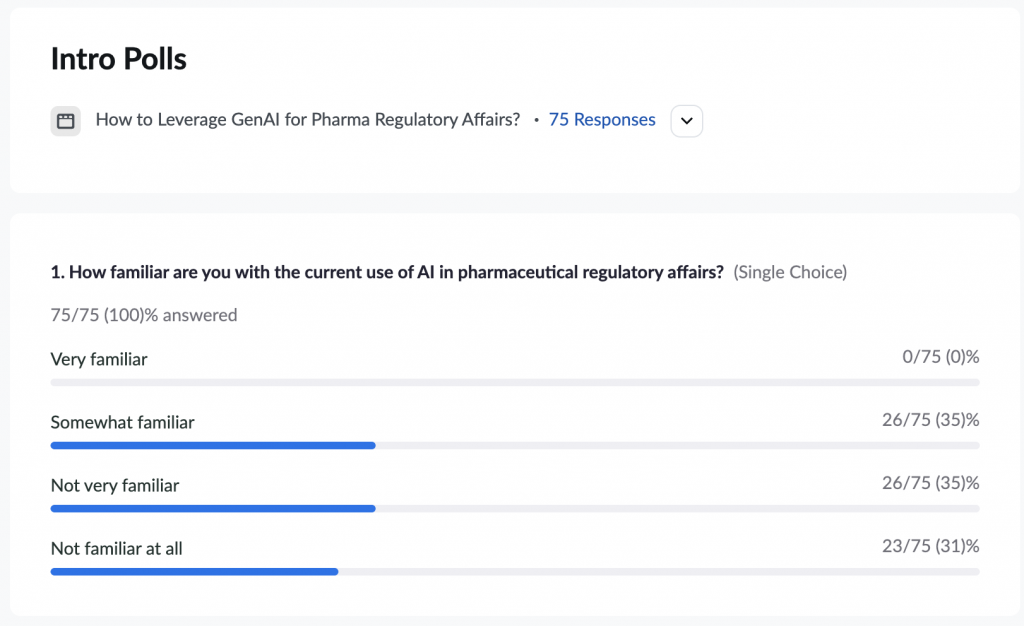
The second poll was on the familiarity of the current use of AI in pharmaceutical regulatory affairs.
Surprisingly, we can see that most of the audience was split between “Somewhat familiar” and “Not very familiar.” And 30% of attendees were not familiar at all!
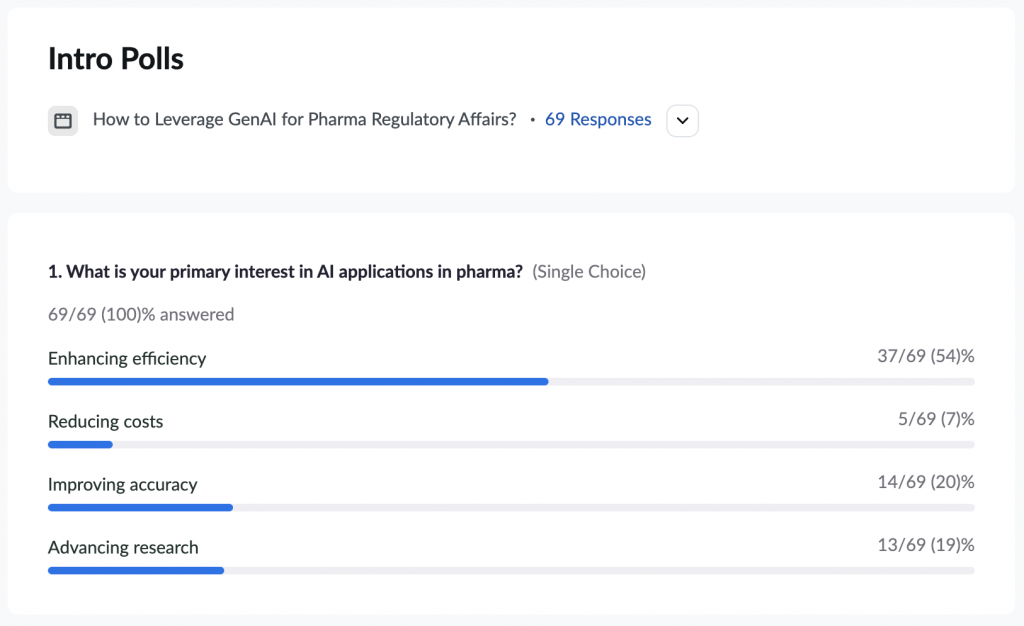
We rolled out the third poll to understand the primary interest of the attendees in AI applications in pharma. Most marked ‘enhancing efficiency’ as their primary interest and the rest got stuck on costs, accuracy, and research.
After a few such polls and based on the answers Santosh started the discussion. Key topics included the integration of AI/ML technologies in regulatory documentation, privacy concerns, and the anonymization process to protect sensitive data. The discussion underscored the need for collaboration between regulatory bodies and companies to adopt new technologies effectively, ensuring data privacy and regulatory compliance. The session also explored the future of AI in pharma, emphasizing cautious optimism and the importance of keeping humans in the loop for decision-making.
Key Takeaways From The Webinar
Data Privacy in Pharma: Critical Clinical Documentation Areas
When asked to highlight the key areas in pharma that need privacy, Scott spoke on the importance of protecting personal and clinical information in regulatory submissions to Health Canada.
It highlights that personal information encompasses any data that can identify an individual, emphasizing the need for privacy in both clinical and non-clinical data. This includes patient information, treatment details, study outcomes, and details about the healthcare professionals involved.
The approach aims to balance transparency with the need to protect identifiable information through anonymization, ensuring that released information provides value without compromising privacy.
Pros and Cons of Patient Data Anonymization
Following the discussion thread, Nutan discussed the pros and cons of anonymizing patient data.
The process of anonymizing personally identifiable information (PII) in documents has evolved from manual to rule-based methods, with each having its own set of pros and cons.
On the one hand, rule-based approaches, while more systematic, struggle to scale with increasing data complexity and volume. On the other hand, analytics and named entity recognition have improved identification accuracy but also introduce challenges in ensuring complete accuracy.
Hybrid models that combine both rule-based and analytical approaches are emerging as a solution, leveraging the strengths of each to enhance data privacy while maintaining operational efficiency. These methods are particularly beneficial in organizations with access to extensive clinical data, allowing for a more nuanced and effective anonymization process.
AI & NLP in Clinical Report Generation
Then Santosh shared the importance of integrating AI & NLP in the regulatory process for Clinical Report Generation:
The industry is highly regulated, emphasizing the importance of having well-established rules and knowledgeable regulatory bodies. Regulations are intentionally slow to adapt to ensure stability and predictability, which is essential for industry trust.
Guidance documents favor principles over prescriptive measures, allowing for multiple compliance methods without altering the fundamental requirements for safety and efficacy. This approach accommodates innovation while maintaining strict standards.
Regulators may remain flexible in their methods, provided the core obligations of safety and efficacy are met, indicating a future where regulatory tools could evolve to further support industry compliance and oversight.
Minimizing AI Risks in Proprietary Data Handling
Santosh asked Nutan about the risk of accidental or malicious exposure of proprietary information by the AI tools. To which Nutan said, “So, the risk associated with technology, particularly in how enterprise search functions can inadvertently expose confidential files due to inadequate access control, rather than an inherent issue with the search technology itself.
It parallels large language models, emphasizing that the risk is more about user access and data handling than the technology. It suggests that comprehensive encryption, selective data use for model training, and robust access controls can mitigate these risks, alongside educating users on secure data practices.“
Impact of GenAI on Clinical Documentation
Santosh asked Nutan about the impact of GenAI on clinical documentation.
Over the past year and a half, Generative AI has significantly impacted clinical documentation by enhancing the contextual understanding and privacy preservation of freestyle documents, such as consent forms.
It excels in distinguishing between medical history and adverse events, making it a valuable tool for identifying personally identifiable information (PII) efficiently. Generative AI’s explainability aids in better decision-making, requiring human oversight to ensure accuracy.
Looking ahead, it promises to revolutionize document generation, raising questions about data permanence and regulatory adaptation to maintain privacy and re-identification risks.
Adoption of GenAI in Pharma
Scott talked about the adoption of GenAI in pharma at the micro level:
Adapting Generative AI in the pharmaceutical industry is expected to start at a micro level, with individuals experimenting with their workflows, leading to broader, industry-wide adoption.
This gradual process will involve building confidence through evaluating benefits versus risks, fostering a slow but steady integration into regulated environments. It’s anticipated that initial resistance will transition into acceptance as standards develop.
Regulators, prioritizing compliance and industry reputation, will likely introduce voluntary standards to guide this integration, ensuring personal information protection and regulatory compliance without altering existing thresholds.
Implementing Privacy-Centric Document Management with GenAI
There is significant interest and concern among pharmaceutical and medical device partners regarding adopting generative AI technologies like ChatGPT, particularly in areas like document processing and privacy. Enterprises are cautious, experimenting with open-source alternatives, and seeking assurances about data privacy and the practical use of large language models.
The conversations have evolved to focus on the importance of contextual understanding for sensitive information in documents, such as differentiating medical history from adverse events, and ensuring safeguards are in place. The discussions also cover the cost-effectiveness and return on investment of using generative AI, highlighting its potential for improving efficiency while maintaining accuracy and privacy.
Top Three GenAI Applications in Pharma Regulatory Affairs
Document Processing and Automation
GenAI can automate the creation, review, and submission of complex regulatory documents in pharmaceutical regulatory affairs. It utilizes advanced algorithms and natural language processing to draft, revise, and ensure documents meet all regulatory standards, significantly reducing manual effort and allowing teams to concentrate on strategic tasks. GenAI’s applications range from automating labeling to ensure consistency across regions to generating safety reports from raw data with high accuracy. Furthermore, it supports the continuous monitoring and updating of documents to comply with changing regulations, marking a shift towards more efficient, accurate, and agile operations in the pharmaceutical industry.
Privacy and Data Protection
GenAI offers a smart approach to data anonymization that addresses the limitations of traditional methods, which often compromise privacy protection or data utility. By generating synthetic data that replicates the statistical characteristics of original datasets without linking records to actual individuals, GenAI ensures privacy in sensitive areas such as clinical trials and patient records. It employs advanced algorithms to create new datasets that are statistically similar to the original but devoid of identifiable information, enhancing privacy protection and reducing re-identification risks. Additionally, GenAI plays a crucial role in regulatory compliance, adapting data handling to evolving privacy laws and proactively identifying potential risks, thereby strengthening privacy protections and simplifying compliance processes for pharmaceutical companies in the complex global regulatory environment.
Regulatory Adaptation
GenAI offers a significant advantage for pharmaceutical companies in adapting to and staying ahead of changing regulatory landscapes. By analyzing vast amounts of regulatory texts, GenAI can swiftly detect changes in requirements and suggest updates to ensure compliance. This real-time adaptability is vital for maintaining market access and ensuring patient safety in a fast-evolving regulatory environment. Additionally, GenAI supports proactive compliance monitoring by scanning for regulatory updates and alerting companies to relevant changes, thereby reducing the risk of penalties. Its predictive capabilities also enable companies to anticipate future regulatory trends, aligning development strategies accordingly for a competitive edge.
Adjusting Re-identification Risk Metrics: How Will Regulatory Bodies Respond?
Nutan asked Scott how the regulatory bodies should respond in adjusting re-identification risk metrics.
Scott explained:
Regulatory agencies might revise re-identification risk thresholds through dialogue and public discourse, ensuring industry adaptation over time. Changes could occur gradually unless prompted by significant events, such as data breaches affecting patient privacy.
Such incidents might prompt immediate, stricter measures before settling into more sustainable practices. Both gradual evolution and responsive adjustments are anticipated, emphasizing transparency and stakeholder engagement in adapting to technological advancements and privacy concerns.
Given the current metrics for the risk of re-identification used by EMA and Health Canada, which differ from approaches by the FDA and other organizations, how might advancements in technology prompt a revision of these standards? Do you anticipate changes to the acceptable thresholds for PII re-identification risks in response to new technological capabilities and the availability of information in the public domain?
Adjustments to the thresholds for privacy and data protection in regulated industries are primarily driven by the need for transparency and involve extensive dialogue and public discourse.
However, should a significant incident such as a data breach occur, immediate and stringent regulatory measures may be implemented to address the situation promptly.
Over time, as technologies evolve and the industry adapts, these standards may change through collaborative discussions or in response to critical events, ensuring that regulations remain effective and relevant.
Conclusion
Generative AI promises to transform regulatory affairs in the pharmaceutical industry, making it more efficient, accurate, and streamlined. However, realizing this potential requires overcoming significant challenges, particularly around data privacy and the need for expertise. As the industry and regulatory bodies adapt to these changes, the focus will remain on ensuring that the integration of AI into regulatory processes enhances compliance and patient safety, marking a new era in pharmaceutical regulation.
Gramener’s expertise in GenAI offers a result-oriented approach to simplifying regulatory affairs in the pharmaceutical industry. By leveraging GenAI, Gramener can automate and streamline complex documentation processes for clinical trials, enhance accuracy in data analysis, and ensure compliance with evolving regulations. Our solutions prioritize data privacy and utilize GenAI’s capabilities to provide insights, making regulatory submissions more efficient and reducing manual efforts significantly. This approach accelerates the regulatory review process and improves overall decision-making in pharmaceutical regulations.
Curious?