We are back with the 6th edition of The AI Chronicles Series. This blog discusses the 2024 Outlook for Life Sciences GenAI, Drug Prices, Regulatory Changes and Challenges, Market Conditions, and Argument- Regulatory Oversight.
Table of Contents
Do check out the previous four editions of the series:
The AI Chronicles #1 – What’s hot in the pharma world with Generative AI, how to build robust patient data privacy, and what’s up with Apple’s AI-powered health coach?
The AI Chronicles #2 – McKinsey’s 5-step digital transformation for Pharma companies, Sanofi’s AI approach to employee productivity, and more on digitization of medical data.
The AI Chronicles #3 – Find content in images and videos with Microsoft’s Vector, Washington’s new health privacy law, and the importance of personalization in healthcare.
The AI Chronicles #4 – AWS’s HealthScribe – a GenAI-powered service, a Roche-sponsored report, and Pytrial – a GenAI tool for various clinical trial tasks
The AI Chronicles #5– GPTs, the EU Clinical Trials Information System (CTIS), Med-PaLM2, ClinicalGPT, PMC-LLAMA, and MedAlpaca.
As we start getting busy in the new year, this first edition of AI Chronicles for 2024 is a special edition to get warmed up for our exciting webinar this month, ‘How to Leverage GenAI for Pharma Regulatory Affairs?’
As the pharma and medical device industry gets more acquainted with the various applications of generative AI, regulatory affairs functions, from regulatory medical writing to regulatory submissions, are getting a lot of attention. While the webinar will get into more depth, this month’s AI Chronicles gives you a sneak peek into some exciting new advances in this field.
2024 Outlook for Life Sciences GenAI, Drug Prices, Economy
What is it about?
The 2024 Outlook for Life Sciences by Deloitte highlights key trends influencing the life sciences sector. It focuses on the anticipated impact of generative AI, the economy, and investment in innovations. The report also discusses workforce and talent challenges, health equity, and supply chain concerns. Biopharma and medical technology companies are expected to prioritize generative AI for research and discovery. Drug pricing, regulatory changes, and market conditions like inflation are among the factors significantly affecting strategies. The outlook suggests a cautiously optimistic view for the life sciences sector in 2024, with a continued focus on innovation and adapting to evolving market conditions.
What does It mean?
As the report re-iterates, more than 90% of biopharma and medtech respondents to the Deloitte survey said they expect generative AI to have an impact on their organizations in 2024. Many companies are exploring ways the technology could automate repetitive back-office functions, reimagine supply chains, or support compliance and regulatory affairs.
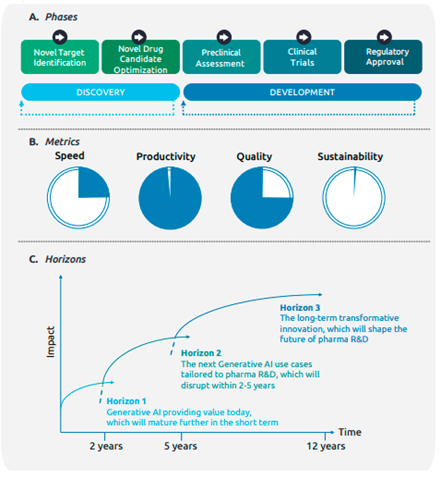
New Horizons: Pioneering Pharmaceutical R&D With Generative AI From Lab to the Clinic — An Industry Perspective
What is it about?
This research paper provides an interesting overview of Generative AI and its role in reduced timescales and reduced dependency on resources. Of particular note is the application for assessing organizational knowledge using virtual assistants.
What does it mean?
The article presents an optimistic view of virtual assistants in pharmaceutical R&D, emphasizing their transformative potential. Large Language Models (LLMs) act as gateways to vast organizational knowledge, streamlining access to crucial data and documents. This accelerates decision-making processes, boosting efficiency and innovation in R&D. Challenges like data privacy and AI biases are acknowledged, but the overall tone is positive, highlighting the future advancements in virtual assistants. These advancements are expected to include enhanced problem-solving capabilities and seamless integration with expert systems, significantly improving productivity and breaking down information barriers in pharmaceutical research.
Regulatory Oversight of Large Language Models (or Generative AI) in Healthcare
What is it about?
This perspective in Nature Digital Medicine provides a different argument- regulatory oversight should assure medical professionals and patients can use LLMs without causing harm or compromising their data or privacy. This paper summarizes practical recommendations by the authors for what we can expect from regulators to bring this vision to reality.
What does it mean?
Of the several regulatory challenges noted by the article, the following three seem to be of utmost importance and urgency. The authors conclude that it is not enough to regulate current LLM models as the new iterations with those advanced capabilities can be expected to get implemented at a similar rate to the previous iterations. Without taking these future additions into consideration, a regulation that focuses on language models could only miss important updates by the time those updates become widely accessible.
| Regulatory challenge | Short description |
| Patient Data Privacy | Ensuring that patient data used for training large language models are fully anonymized and protected from potential breaches. This poses a significant regulatory challenge, as any violation could lead to serious consequences under privacy laws like HIPAA in the US. |
| Intellectual Property | If an LLM generates content similar to proprietary medical research or literature, it could lead to issues regarding intellectual property rights. |
| Interpretability & Transparency | Regulations need to ensure transparency about how decisions are made by the AI. This is particularly challenging with AI models that are often termed as “black boxes” due to their complex algorithms. |
| Continuous Monitoring & Validation | Ensuring the continuous performance, accuracy, and validity of AI tools over time and across different populations is a critical regulatory challenge. |
The Gramener Take
As an advanced analytics and AI provider, Gramener is well placed to leverage these trends by offering tailored solutions in data analytics and AI to life sciences companies. Our capabilities in areas from drug development to commercial pharma and Gen AI for data-driven decision-making are particularly relevant. Our capabilities in Generative AI, machine learning, and data visualization help in enhancing virtual assistant functionalities, such as integrating with internal databases, improving decision-making processes, and streamlining R&D operations. Gramener’s solutions also address challenges outlined in the paper, like data privacy and AI biases. By aligning with the life sciences sector’s growing demand for AI-driven research and development, we hope to play a crucial role in accelerating innovation and efficiency in this field. In the upcoming webinar, we will touch upon many of these challenges as well as opportunities from these exciting advances in the field of generative AI.